Topic: Gliding reptiles
In the reptiles, different forms of skin membrane (called ‘patagia’) and in some extinct species, primitive feathers, have evolved convergently as adaptations for gliding.
The ability to glide has evolved independently in a surprising diversity of animals, including reptiles, mammals, frogs, ants, fish and even in some species of squid. Gliding is defined as descent through the air at an angle of less than 45° to the horizontal, and this mode of locomotion has been achieved through a range of impressive morphological and behavioural adaptations that generate the required aerodynamic forces (upward ‘lift’ exceeding air resistance, or ‘drag’).
Among the distinct types of gliding that are known, several provide excellent case studies in convergent evolution, as they have evolved repeatedly in unrelated groups. Taking reptiles as our current example, different forms of skin membrane (called ‘patagia’) and in some extinct species, primitive feathers, have evolved convergently as adaptations for gliding – appearing in the so-called ‘lepidosaurs’, including groups such as the squamates (lizards, snakes and amphisbaenians) and extinct marine reptiles, as well as the ‘archosaurs’, which include groups such as crocodiles and most famously, the dinosaurs.
1. Reptilian gliding: inter-digital membranes
 One of the main adaptations for gliding in certain tree-dwelling lizards is the presence of skin membranes (‘patagia’) on various parts of the body, that open out passively due to air pressure as the animal falls, generating a degree of lift. In addition, the body is often highly flattened and stereotyped body movements may be employed to ensure an efficient and stable glide. Well-developed patagial membranes between fingers and toes (technically termed ‘digits’) are a prime feature of gliding geckos (e.g. two species of the gecko genus Cosymbotus and six species of Ptychozoon from South-East Asia and Indo-Australia) and the distantly related West African lacertid, Holaspis guentheri. Convergence on webbed digits and stereotyped gliding postures has occurred not only in lizards (geckos and lacertids), but also in (i) two independent lineages of ‘flying’ frogs (New World ‘Hylidae’ e.g. Hyla miliaria and Old World ‘Rhacophoridae’ e.g. Rhacophorus nigropalmatus, Polypedates dennysi) from tropical forest environments, and (ii) colugos which are extremely specialised gliding mammals. The two known species of colugo (Cynocephalus volans and Galeopterus varieagatus) inhabit S.E. Asian rainforests, and may be the closest living relatives of primates. Frogs use gliding to reach mating sites on the rainforest floor and to escape from predators, whereas geckos, Holaspis and colugos primarily glide between trees in search of food, mates and secondarily safety from predation.
One of the main adaptations for gliding in certain tree-dwelling lizards is the presence of skin membranes (‘patagia’) on various parts of the body, that open out passively due to air pressure as the animal falls, generating a degree of lift. In addition, the body is often highly flattened and stereotyped body movements may be employed to ensure an efficient and stable glide. Well-developed patagial membranes between fingers and toes (technically termed ‘digits’) are a prime feature of gliding geckos (e.g. two species of the gecko genus Cosymbotus and six species of Ptychozoon from South-East Asia and Indo-Australia) and the distantly related West African lacertid, Holaspis guentheri. Convergence on webbed digits and stereotyped gliding postures has occurred not only in lizards (geckos and lacertids), but also in (i) two independent lineages of ‘flying’ frogs (New World ‘Hylidae’ e.g. Hyla miliaria and Old World ‘Rhacophoridae’ e.g. Rhacophorus nigropalmatus, Polypedates dennysi) from tropical forest environments, and (ii) colugos which are extremely specialised gliding mammals. The two known species of colugo (Cynocephalus volans and Galeopterus varieagatus) inhabit S.E. Asian rainforests, and may be the closest living relatives of primates. Frogs use gliding to reach mating sites on the rainforest floor and to escape from predators, whereas geckos, Holaspis and colugos primarily glide between trees in search of food, mates and secondarily safety from predation.
 Augmenting inter-digital webbing, gliding geckos and lacertids have small lateral fringes or flaps on the digits, limbs, trunk and tail; these patagia enhance aerofoil properties when gliding from tree to tree, as well as functioning in camouflage (‘crypsis’) when the animal is motionless. Small gliding membranes on the limbs and body trunk are convergent features that also characterise several gliding frogs (e.g. Rhacophorus nigropalmatus, R. malabaricus, R. pardalis), and are reminiscent of the more extensive ‘wing’ patagia found in certain extinct reptiles (e.g. Coelurosauravus jaekeli, Xianglong zhaoi, Sharovipteryx mirabilis) and gliding mammals (e.g. flying squirrels, phalangers, colugos).
Augmenting inter-digital webbing, gliding geckos and lacertids have small lateral fringes or flaps on the digits, limbs, trunk and tail; these patagia enhance aerofoil properties when gliding from tree to tree, as well as functioning in camouflage (‘crypsis’) when the animal is motionless. Small gliding membranes on the limbs and body trunk are convergent features that also characterise several gliding frogs (e.g. Rhacophorus nigropalmatus, R. malabaricus, R. pardalis), and are reminiscent of the more extensive ‘wing’ patagia found in certain extinct reptiles (e.g. Coelurosauravus jaekeli, Xianglong zhaoi, Sharovipteryx mirabilis) and gliding mammals (e.g. flying squirrels, phalangers, colugos).
Another very remarkable instance where lateral body flaps, or flanges, have evolved to assist controlled gliding occurs in a number of ants inhabiting tropical forests. Tree-dwelling ants capable of controlled gliding when dislodged or threatened by predation include members of the families Myrmicinae (e.g. Cephalotus atratus, Daceton armigerum, Procryptocerus convergens), Pseudomyrmecinae (e.g. Pseudomyrmex elongatus) and Formicinae (a single species, Camponotus canescens). These ants are further adapted to gliding by having a relatively dorso-ventrally flattened body for an improved lift:drag ratio (convergent with gliding lizards), good vision for locating landing sites, and – especially in the Myrmicinae and Pseudomyrmicini which glide abdomen first – a moveable post-petiole region, enabling steering during aerial descent.
 As a final example of possible convergent recruitment of lateral body flaps to aid gliding, there is a (somewhat tentative) connection between flap-mediated gliding in reptiles and the 15 or so known species of ‘flying’ squid. These marine creatures are cephalopod molluscs (i.e. invertebrates) of the family Ommatostrephidae, and include species such as the Humboldt squid Dosidicus gigas, Japanese flying squid Todarodes pacificus and neon flying squid Ommastrephes bartramii. Aerial locomotion in squid depends mainly on powerful jet propulsion as water is forced out of a modified siphon, but well-developed lateral fins are proposed to play a role in stabilising and directing its gliding flight, reminiscent of the likely role of lateral body fringes in squamates such as Ptychozoon and Holaspis in conferring extra lift and stability during descent.
As a final example of possible convergent recruitment of lateral body flaps to aid gliding, there is a (somewhat tentative) connection between flap-mediated gliding in reptiles and the 15 or so known species of ‘flying’ squid. These marine creatures are cephalopod molluscs (i.e. invertebrates) of the family Ommatostrephidae, and include species such as the Humboldt squid Dosidicus gigas, Japanese flying squid Todarodes pacificus and neon flying squid Ommastrephes bartramii. Aerial locomotion in squid depends mainly on powerful jet propulsion as water is forced out of a modified siphon, but well-developed lateral fins are proposed to play a role in stabilising and directing its gliding flight, reminiscent of the likely role of lateral body fringes in squamates such as Ptychozoon and Holaspis in conferring extra lift and stability during descent.
2. Limb-associated ‘wing’ membranes
Sharovipteryx is a small reptile from the Upper Triassic (225 million years ago), and was evidently tree-dwelling (‘arboreal’). Presumably it was able to run up trees aided by claws on its two back legs, and then launch itself into a powerful, controlled glide. Its gliding mechanism is unique among the reptiles: a wide, triangular membrane (termed a ‘delta wing‘) spreads between its outstretched hindlimbs at the posterior and joins the body just behind the shoulders to form an anterior point. In addition to the primary ‘delta wing’ Sharovipteryx is hypothesised to have had small membranes between the forelimbs and base of the neck, providing enhanced maneuverability. Sharovipteryx is an enigmatic reptile, probably belonging to the primitive archosaur group ‘Prolacertiformes’. Notably, gliding geckos and lacertids are only very distantly related, being members of the other major group, the lepidosaurs. Delta wing gliding is most similar to that seen in gliding mammals, where passive wing patagia extending from the limbs and body have evolved in spite of hundreds of millions of years of independent evolution between the two groups. Gliding mammals are remarkably diverse, and typically have a patagium extending from the forelimb ‘elbow’ to the hindlimb ‘ankle’ or tail region, making an effective structure for maximising lift and minimising drag. Gliding has evolved independently at least seven times in the mammals, making them an interesting example of convergent evolution in their own right!
 Among the placental mammals two rodent groups,’flying’ squirrels (Sciuridae: Pteromyini) and anomalures, or scaly-tailed ‘flying squirrels’ (Anomaluridae) have independently acquired a patagium that reaches from the wrist to the ankle, is controlled by cartilage spurs, and is enhanced as a gliding device by a flattened, broad tail. In the anomalures, two rows of scales are also present under the tail, possibly providing extra lift during gliding. Gliding squirrels are widespread in Eurasia and North America, and comprise 15 genera, including Petaurista (giant flying squirrels) in S.E. Asia and Glaucomys in N. America. Anomalures inhabit forests of Central Africa, and include the genera Anomalurus (e.g. A. beecrofti, Beecroft’s flying ‘squirrel’), Idiurus (e.g. I. macrotis, long-eared flying ‘mouse’) and Zenkerella.
Among the placental mammals two rodent groups,’flying’ squirrels (Sciuridae: Pteromyini) and anomalures, or scaly-tailed ‘flying squirrels’ (Anomaluridae) have independently acquired a patagium that reaches from the wrist to the ankle, is controlled by cartilage spurs, and is enhanced as a gliding device by a flattened, broad tail. In the anomalures, two rows of scales are also present under the tail, possibly providing extra lift during gliding. Gliding squirrels are widespread in Eurasia and North America, and comprise 15 genera, including Petaurista (giant flying squirrels) in S.E. Asia and Glaucomys in N. America. Anomalures inhabit forests of Central Africa, and include the genera Anomalurus (e.g. A. beecrofti, Beecroft’s flying ‘squirrel’), Idiurus (e.g. I. macrotis, long-eared flying ‘mouse’) and Zenkerella.
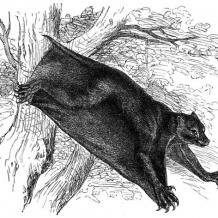 The extreme mammalian adaptation to gliding is found in the colugos (Dermoptera: Cynocephalidae), which are nocturnal tree-dwellers capable of gliding up to 70m between trees without any significant loss of height. Cynocephalus volans and Galeopterus variegatus inhabit S.E. Asian rainforests, and two fossil colugo species (Dermotherium major and D. chimaera) are known. The colugo patagium covers as great a surface area as geometrically possible, its light, long-limbed skeletal structure is adapted for maximising lift, and it possesses excellent binocular vision for precision landing. Within the closely related primates we find limited gliding ability in Madagascan lemurs (family Indriiidae) of the genus Propithecus, commonly known as sifaka. Propithecus diadema and its allies have thick hair on the forearm and a small membrane on inner forelimb, both of which provide limited aerofoil properties and degree of lift when jumping between the trees. The only other known gliding placental mammal is an extinct species, Volaticotherium antiquus, known from Lower Cretaceous (~125Ma) rocks of Inner Mongolia. It was a small insectivore (12-14cm long), similar in mass to the flying squirrel Glaucomys volans, and had a furry patagium stretched between fore- and hind-limbs, a smaller membrane (‘uropatagium’) between the hindlimbs and tail, and both elongated limbs and a stiff, flattened tail for stability and control.
The extreme mammalian adaptation to gliding is found in the colugos (Dermoptera: Cynocephalidae), which are nocturnal tree-dwellers capable of gliding up to 70m between trees without any significant loss of height. Cynocephalus volans and Galeopterus variegatus inhabit S.E. Asian rainforests, and two fossil colugo species (Dermotherium major and D. chimaera) are known. The colugo patagium covers as great a surface area as geometrically possible, its light, long-limbed skeletal structure is adapted for maximising lift, and it possesses excellent binocular vision for precision landing. Within the closely related primates we find limited gliding ability in Madagascan lemurs (family Indriiidae) of the genus Propithecus, commonly known as sifaka. Propithecus diadema and its allies have thick hair on the forearm and a small membrane on inner forelimb, both of which provide limited aerofoil properties and degree of lift when jumping between the trees. The only other known gliding placental mammal is an extinct species, Volaticotherium antiquus, known from Lower Cretaceous (~125Ma) rocks of Inner Mongolia. It was a small insectivore (12-14cm long), similar in mass to the flying squirrel Glaucomys volans, and had a furry patagium stretched between fore- and hind-limbs, a smaller membrane (‘uropatagium’) between the hindlimbs and tail, and both elongated limbs and a stiff, flattened tail for stability and control.
Among the marsupials gliding has evolved independently on three separate occasions: in the gliding possums, greater glider and feather-tailed possums. Gliding possums or ‘phalangers’ comprise six species in the genus Petaurus (family Petauridae), most well known for the Biak glider (P. biacensis) and Sugar glider (P. breviceps). Petaurids inhabit forests of Australia, New Guinea and Borneo, and are able to glide between trees for over 50m in search of sap, nectar or small insects to eat. They employ a patagium that is very similar to the eutherian group Pteromyini (flying squirrels). Stabilisation during gliding depends on a broad, flattened tail, as in most other flying mammals, and also in gliding lizards (e.g. Ptychozoon, Holaspis) to a degree.  The greater glider Petauroides volans belongs to the family Pseudochieridae, and resembles Petaurus in its habits and form, except that the patagium is more restricted, stretching from the fifth finger to the hindlimb ‘knee’ rather than first toe. Feather-tailed possums refer to two species of the family Acrobatidae, including the smallest known gliding mammal, the Australian ‘feather-tailed glider’ Acrobates pygmaeus, and the feather-tailed possum of New Guinea, Distoechurus pennatus. Both have a patagium between the fore- and hind-limbs, and are uniquely characterised by a tail bearing long, stiffened hairs running down both sides, giving it a feather-like appearance and providing good steering ability when gliding.
The greater glider Petauroides volans belongs to the family Pseudochieridae, and resembles Petaurus in its habits and form, except that the patagium is more restricted, stretching from the fifth finger to the hindlimb ‘knee’ rather than first toe. Feather-tailed possums refer to two species of the family Acrobatidae, including the smallest known gliding mammal, the Australian ‘feather-tailed glider’ Acrobates pygmaeus, and the feather-tailed possum of New Guinea, Distoechurus pennatus. Both have a patagium between the fore- and hind-limbs, and are uniquely characterised by a tail bearing long, stiffened hairs running down both sides, giving it a feather-like appearance and providing good steering ability when gliding.
3. Rod-supported ‘wing’ membranes
The agamid lizard genus Draco (‘flying dragon’) and four lineages of extinct reptiles have each independently evolved musculo-skeletal adaptations for gliding on a pair of flight membranes (patagia) that expand laterally from the dorsal trunk region. These lateral patagia are supported by rod-like structures made of bone or dermal deposits, and they are unfolded through the action of specialised muscles and ligaments.
 In Draco, elongated dorsal thoracic ribs support a broad patagium that flares outwards between the fore- and hind-limbs; membrane extension and movement during flight is under voluntary control, dependent on rib-associated muscles and ligaments. Draco glides from tree to tree in search of food, mates, to chase territorial males, and to avoid predators; smaller species (e.g. D. melanopogon, D. quinquefasciatus of Borneo) tend to occupy lower levels of the trees, as their large wing surface area : body mass ratio requires a shorter initial ‘ballistic dive’ before achieving enough lift for a level glide, whereas larger species (e.g. D. fimbriatus) are high canopy specialists, requiring an initial launch from higher up to generate enough lift to carry their greater body mass.
In Draco, elongated dorsal thoracic ribs support a broad patagium that flares outwards between the fore- and hind-limbs; membrane extension and movement during flight is under voluntary control, dependent on rib-associated muscles and ligaments. Draco glides from tree to tree in search of food, mates, to chase territorial males, and to avoid predators; smaller species (e.g. D. melanopogon, D. quinquefasciatus of Borneo) tend to occupy lower levels of the trees, as their large wing surface area : body mass ratio requires a shorter initial ‘ballistic dive’ before achieving enough lift for a level glide, whereas larger species (e.g. D. fimbriatus) are high canopy specialists, requiring an initial launch from higher up to generate enough lift to carry their greater body mass.
Dorsal ribs have been found to support gliding membranes in several extinct reptiles, including the basal squamate Xianglong zhaoi (Early Cretaceous), late Triassic lepidosaurs Kuehneosaurus and Icarosaurus siefkeri, and the very distantly related mid-Triassic (220 Ma) archosaur Mecistotrachelos apeoros.  A late Permian lepidosaur has been identified as gliding on a rod-supported patagium, but in this species – Coelurosauravus jaekeli – the support structure is not rib-derived, but made of bony material deposited within the skin. Our understanding of reptile relationships reveals that these reptiles all evolved rod-supported patagia independently, a conclusion supported by observed variation in patagium outline, aerodynamic properties and precise musculo-skeletal arrangements between species. Indeed, the extinct Xianglong exhibits possibly the most aerodynamic and maneuverable rib-supported patagium, with a typical aerofoil cross-section (thickened at the leading edge, tapered to the trailing edge) and wings that were much longer laterally than antero-posteriorly (in contract to Draco, for example, whose ‘wing’ patagium is longer in the anterior-posterior dimension than transversely). Furthermore, Mecistotrachelos was probably more maneuverable than Draco, owing to thickened elongated ribs near the base of its unusually long neck, suggestive of strong muscle attachment for excellent control over its membranous ‘wings’.
A late Permian lepidosaur has been identified as gliding on a rod-supported patagium, but in this species – Coelurosauravus jaekeli – the support structure is not rib-derived, but made of bony material deposited within the skin. Our understanding of reptile relationships reveals that these reptiles all evolved rod-supported patagia independently, a conclusion supported by observed variation in patagium outline, aerodynamic properties and precise musculo-skeletal arrangements between species. Indeed, the extinct Xianglong exhibits possibly the most aerodynamic and maneuverable rib-supported patagium, with a typical aerofoil cross-section (thickened at the leading edge, tapered to the trailing edge) and wings that were much longer laterally than antero-posteriorly (in contract to Draco, for example, whose ‘wing’ patagium is longer in the anterior-posterior dimension than transversely). Furthermore, Mecistotrachelos was probably more maneuverable than Draco, owing to thickened elongated ribs near the base of its unusually long neck, suggestive of strong muscle attachment for excellent control over its membranous ‘wings’.
 Beyond general comparisons of gliding patagia, the Cretaceous lizard Xianglong points to a more subtle instance of convergent evolution, as it strengthens its patagium with parallel collagen fibres in a manner only seen elsewhere in very distantly related taxa, namely the pterosaurs (capable of true flight) and the Triassic delta-wing glider Sharovipteryx, indicating that these three reptile groups independently converged on the use of collagen fibres to critically reinforce flight membranes.
Beyond general comparisons of gliding patagia, the Cretaceous lizard Xianglong points to a more subtle instance of convergent evolution, as it strengthens its patagium with parallel collagen fibres in a manner only seen elsewhere in very distantly related taxa, namely the pterosaurs (capable of true flight) and the Triassic delta-wing glider Sharovipteryx, indicating that these three reptile groups independently converged on the use of collagen fibres to critically reinforce flight membranes.
 In discussing reptiles with rib-supported patagia, it is impossible to avoid a most fascinating group from the forests of S.E. Asia, namely the gliding tree snakes. Two species of gliding tree snake have been well studied; they are from the group of snakes known as colubrids, and include the paradise tree snake Chrysopelea paradisi, and the golden tree snake C. ornata. These snakes use a uniquely specialised body shape and aerial behaviour to produce controlled glides that equal the performance of the gliding agamid Draco melanopogon. The research of J. J. Socha has shown that Chrysopelea paradisi achieves lift, after jumping into a dive from a perch in the trees, by passing “lateral travelling waves posteriorly along a long dorsoventrally flattened body”. During descent the ribs are splayed outwards so that the ventral (lower) surface is concave, and in combination with stereotypic lateral undulations, the whole body effectively functions as a single, aerodynamic wing. C. paradisi has more concave ventral surface than C. ornata, and it also has lower body mass for its length, making it a better glider than C. ornata, capable of gliding more than twice as far between the trees, chasing prey or avoiding predation. Although not structurally identical to Draco-type gliding lizards, the principle of using expanded ribs is the same, representing a convergent adaptation for generating lift.
In discussing reptiles with rib-supported patagia, it is impossible to avoid a most fascinating group from the forests of S.E. Asia, namely the gliding tree snakes. Two species of gliding tree snake have been well studied; they are from the group of snakes known as colubrids, and include the paradise tree snake Chrysopelea paradisi, and the golden tree snake C. ornata. These snakes use a uniquely specialised body shape and aerial behaviour to produce controlled glides that equal the performance of the gliding agamid Draco melanopogon. The research of J. J. Socha has shown that Chrysopelea paradisi achieves lift, after jumping into a dive from a perch in the trees, by passing “lateral travelling waves posteriorly along a long dorsoventrally flattened body”. During descent the ribs are splayed outwards so that the ventral (lower) surface is concave, and in combination with stereotypic lateral undulations, the whole body effectively functions as a single, aerodynamic wing. C. paradisi has more concave ventral surface than C. ornata, and it also has lower body mass for its length, making it a better glider than C. ornata, capable of gliding more than twice as far between the trees, chasing prey or avoiding predation. Although not structurally identical to Draco-type gliding lizards, the principle of using expanded ribs is the same, representing a convergent adaptation for generating lift.
4. Feathered wings and tails
 A number of reptile species have been discovered in the Mesozoic fossil record, bearing feathers that were apparently used to support gliding locomotion, rather than true flight as in present day birds. Feathered gliders were all archosaurs, unrelated to modern lizards but instead identified as a group of dinosaurs called the theropods, out of which emerged the lineage(s) leading to modern birds (technically termed ‘Aves’). Mesozoic gliding theropods are all (or almost all) from the advanced group ‘Maniraptora’ but belong to different sub-groups (Oviraptorosauria and Deinonychosauria), indicating convergent evolution of feathers for gliding.
A number of reptile species have been discovered in the Mesozoic fossil record, bearing feathers that were apparently used to support gliding locomotion, rather than true flight as in present day birds. Feathered gliders were all archosaurs, unrelated to modern lizards but instead identified as a group of dinosaurs called the theropods, out of which emerged the lineage(s) leading to modern birds (technically termed ‘Aves’). Mesozoic gliding theropods are all (or almost all) from the advanced group ‘Maniraptora’ but belong to different sub-groups (Oviraptorosauria and Deinonychosauria), indicating convergent evolution of feathers for gliding.
 As an aside, there is great controversy about the origins of true flight and birds, although it is generally agreed to have evolved from feathered, reptilian ancestors. It is notable that the existence of multiple lineages of tree-dwelling, feathered gliders, not to mention terrestrial, feathered ‘runners’, may leave space for multiple possible ancestors. For example, the famous bird-lizard Archaeopteryx lithographica of the Late Jurassic (155-150 Ma) was apparently capable of true flight and is widely considered to be a true bird ancestor in the group ‘Avialae’, but another species in the closely related group Oviraptosauria, Avimimus portentosus of the Late Cretaceous (75 Ma), has also been proposed as the ‘closest link’ between reptiles and birds, having feathered wings used not in flight but as an aid in specialised running.
As an aside, there is great controversy about the origins of true flight and birds, although it is generally agreed to have evolved from feathered, reptilian ancestors. It is notable that the existence of multiple lineages of tree-dwelling, feathered gliders, not to mention terrestrial, feathered ‘runners’, may leave space for multiple possible ancestors. For example, the famous bird-lizard Archaeopteryx lithographica of the Late Jurassic (155-150 Ma) was apparently capable of true flight and is widely considered to be a true bird ancestor in the group ‘Avialae’, but another species in the closely related group Oviraptosauria, Avimimus portentosus of the Late Cretaceous (75 Ma), has also been proposed as the ‘closest link’ between reptiles and birds, having feathered wings used not in flight but as an aid in specialised running.
Feathered Wings
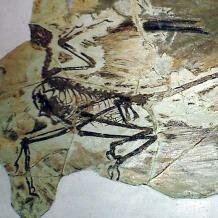 Within the last decade, a number of astonishingly preserved Mesozoic reptiles bearing bird-like traits have been unearthed in China. Two important examples among these are the small dromeosaur Microraptor gui (Early Cretaceous, 130-125 Ma) and the earlier archosaur Longisquama insignis (Late Triassic, 220 Ma). Both of these reptiles evolved heavily feathered bodies as a convergent adaptation to gliding between trees. As a dromeosaur, Microraptor is a member of the ‘Deinonychosauria’, adjacent to the bird-containing group Avialae, and it possesses feathers with asymmetric vanes (as in birds) arranged to form paired forewings and hindwings, with a terminal diamond-shaped fan on the tail. Longisquama was adapted to gliding with scaly, overlapping ‘feathers’ arranged as an ideal aerofoil on the forelimb, paired clusters along the dorsal surface (back) of the body and as tufts on the chin and neck. The feathers of L. insignis may or may not be equivalent to those observed in Microraptor and Archaeopteryx: their detailed structure, and the relationship of Longisquama to other dinosaurs, is still uncertain. The bird-like and impressively aerodynamic morphologies of Microraptor and Longisquama have led to the suggestion that both could be bird ancestors, meaning that avian flight would not represent a single innovation, but evolved by convergence on separate occasions.
Within the last decade, a number of astonishingly preserved Mesozoic reptiles bearing bird-like traits have been unearthed in China. Two important examples among these are the small dromeosaur Microraptor gui (Early Cretaceous, 130-125 Ma) and the earlier archosaur Longisquama insignis (Late Triassic, 220 Ma). Both of these reptiles evolved heavily feathered bodies as a convergent adaptation to gliding between trees. As a dromeosaur, Microraptor is a member of the ‘Deinonychosauria’, adjacent to the bird-containing group Avialae, and it possesses feathers with asymmetric vanes (as in birds) arranged to form paired forewings and hindwings, with a terminal diamond-shaped fan on the tail. Longisquama was adapted to gliding with scaly, overlapping ‘feathers’ arranged as an ideal aerofoil on the forelimb, paired clusters along the dorsal surface (back) of the body and as tufts on the chin and neck. The feathers of L. insignis may or may not be equivalent to those observed in Microraptor and Archaeopteryx: their detailed structure, and the relationship of Longisquama to other dinosaurs, is still uncertain. The bird-like and impressively aerodynamic morphologies of Microraptor and Longisquama have led to the suggestion that both could be bird ancestors, meaning that avian flight would not represent a single innovation, but evolved by convergence on separate occasions.
 ‘Four-winged’ gliding, as in Microraptor, is reminiscent of the mechanism used by ‘flying’ fish, even in their capacity to actively move their ‘wings’, giving the impression of a transition between gliding and true, powered flight. Flying fish comprise approximately 50 species of the fish family Exocoetidae (e.g. Cheilopogon, Exocoetus, Hirundichthys), and a few species of the closely related Hemirhamphidae (e.g. Euleptorhamphus viridis), all inhabiting tropical and subtropical waters of the Atlantic, Pacific and Indian Oceans . They travel on average 30-50m per glide, twice as fast as they are able to swim (up to 60km/hr), and manage to do so by spreading (and flapping) their enlarged pectoral and pelvic fins after launching themselves from the water using forward thrust from a deeply forked, fast-moving caudal (or ‘tail’) fin. Four-winged exocoetid gliding parallels a general mechanism shared with feathered, four-winged Microraptor, in which the fore- and hind-limbs are modified into effective aerofoil structures for aerial locomotion. Furthermore, two-winged species of exocoetid that glide only on enlarged pectoral (forelimb) fins effectively mirror the mode of gliding found in Longisquama, with its ‘feathered’ forelimb wings and tail fan. These are remarkable examples of convergent evolution between reptiles and fish, two vertebrate groups that have been evolving separately for hundreds of millions of years.
‘Four-winged’ gliding, as in Microraptor, is reminiscent of the mechanism used by ‘flying’ fish, even in their capacity to actively move their ‘wings’, giving the impression of a transition between gliding and true, powered flight. Flying fish comprise approximately 50 species of the fish family Exocoetidae (e.g. Cheilopogon, Exocoetus, Hirundichthys), and a few species of the closely related Hemirhamphidae (e.g. Euleptorhamphus viridis), all inhabiting tropical and subtropical waters of the Atlantic, Pacific and Indian Oceans . They travel on average 30-50m per glide, twice as fast as they are able to swim (up to 60km/hr), and manage to do so by spreading (and flapping) their enlarged pectoral and pelvic fins after launching themselves from the water using forward thrust from a deeply forked, fast-moving caudal (or ‘tail’) fin. Four-winged exocoetid gliding parallels a general mechanism shared with feathered, four-winged Microraptor, in which the fore- and hind-limbs are modified into effective aerofoil structures for aerial locomotion. Furthermore, two-winged species of exocoetid that glide only on enlarged pectoral (forelimb) fins effectively mirror the mode of gliding found in Longisquama, with its ‘feathered’ forelimb wings and tail fan. These are remarkable examples of convergent evolution between reptiles and fish, two vertebrate groups that have been evolving separately for hundreds of millions of years.
Feathered tails
 Some 10- 25 Ma before the appearance of four-winged Microraptor in the fossil record, we find evidence of some interesting gliding theropods in a group known as the ‘Oviraptorosauria’. Oviraptorosaurs are slightly less evolutionarily advanced than the so-called ‘Eumaniraptoria’ – which comprise the Deinonychosauria (e.g. Microraptor and Sinovenator, capable of gliding) and Avialae (e.g. Archaeopteryx, Jeholornis and Sapeornis, primitive ‘birds’ capable of flight). The oviraptor Protarchaeopteryx robusta had indisputably bird-like feathers as a tuft on the end of its tail, and Caudipteryx zoui had a well feathered tail and forelimbs; both species are Late Jurassic-Early Cretaceous (150-140 Ma) in age.
Some 10- 25 Ma before the appearance of four-winged Microraptor in the fossil record, we find evidence of some interesting gliding theropods in a group known as the ‘Oviraptorosauria’. Oviraptorosaurs are slightly less evolutionarily advanced than the so-called ‘Eumaniraptoria’ – which comprise the Deinonychosauria (e.g. Microraptor and Sinovenator, capable of gliding) and Avialae (e.g. Archaeopteryx, Jeholornis and Sapeornis, primitive ‘birds’ capable of flight). The oviraptor Protarchaeopteryx robusta had indisputably bird-like feathers as a tuft on the end of its tail, and Caudipteryx zoui had a well feathered tail and forelimbs; both species are Late Jurassic-Early Cretaceous (150-140 Ma) in age.
A dominant tail plume is hypothesised to stabilise gliding between trees, and as such represents an astonishing convergence with the morphological and functional adaptations of certain gliding mammals. For example, gliding in scaly-tailed anomalures (African rodents – e.g. Anomalurus, Idiurus) and feather-tailed possums (marsupials of the family Acrobatidae – e.g. Acrobates, Distoechurus) is dependent on either projecting rows of scales or stiff, feather-like hairs respectively, each located on either side of a flattened, broad tail. On a smaller evolutionary scale, Caudipteryx and Protarchaeopteryx evolved a ‘tail-wing’ independently from one another within the Oviraptorosauria, representing convergent evolution of a gliding adaptation that appears to be only a small step behind the specialised ‘four-wings plus tail-fan’ of Microraptor.
Cite this web page
Map of Life - "Gliding reptiles"
https://mapoflife.org/topics/topic_342_gliding-reptiles/
April 22, 2021

